Abstract
Background and Purpose: Subsequent malignant neoplasms (SMNs) are a leading cause of non-relapse mortality after blood or marrow transplantation (BMT). Cancers of the gastrointestinal (GI) tract are of special interest since their clinical behavior is often aggressive, but early detection through screening is possible for specific GI malignancies. There is limited information regarding the risk of GI SMNs following BMT. The purpose of this study was to report the risk of GI SMNs after BMT and to identify specific exposures associated with increased risk.
Materials and Methods: The BMT Survivor Study (BMTSS) is a multi-institutional retrospective cohort study of patients transplanted between 1974 and 2014, with survival ≥2y. Disease and treatment characteristics were obtained from institutional databases and medical records. GI SMNs were identified by review of the BMTSS survey or death records and confirmed by pathology report and/or medical record review. Standardized incidence ratios (SIRs) were calculated to determine excess risk of GI SMNs compared with the general population. Fine-Gray competing risks models examined the association between treatment exposures and GI SMNs.
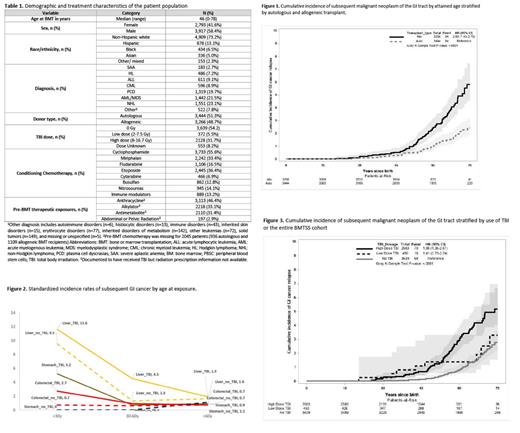
Results: Clinical/demographic characteristics of the cohort are provided in Table 1. The 6,710 BMT recipients in this cohort contributed 62,479 person-years of follow-up, yielding 148 patients with GI SMNs occurring a median of 8.9y (range, 0.3-36.6y) from BMT. The GI SMNs in the cohort included colorectal (n=45), liver (n=36), pancreatic (n=27), esophageal (n=22), gastric (n=11), and other GI (n=7) cancers. By age 70, the cumulative incidence of a GI SMN was 4.8% for allogeneic BMT recipients vs. 1.9% for autologous BMT recipients (p<0.0001, Fig 1). The SIR for a GI SMN after BMT was 1.95 for allogeneic BMT recipients vs. 1.03 for autologous BMT recipients (p=0.8). The risk of esophageal (SIR=3.19, p<0.01), liver (SIR=3.06, p<0.01) and pancreatic cancer (SIR=1.79, p<0.01) was increased when compared to the general population whereas the risk for gastric (SIR=1.15, p=0.6) and colorectal cancers (SIR=0.86, p=0.3) was not. Radiation exposure: Exposure to TBI at age <30y was associated with significantly higher risk for colorectal (SIR=2.67, p=0.003), stomach (SIR=5.16, p=0.005), and liver (SIR=11.61, p<0.001) cancers; the risks were not elevated when TBI exposure occurred at age >60 (Fig 2). The cumulative incidence of GI SMNs by age 70 was significantly higher (p<0.0001) among patients who received high-dose TBI (≥8Gy: 4.8%) vs. those who received low-dose TBI (<8Gy: 2.5%) or no TBI (2.1%) (Fig 3). High-dose TBI (and not low-dose) was associated with a 2.5-fold higher risk of colorectal cancer (95%CI=1.3-4.7). Pre-BMT abdominal radiation was associated with 9.55-fold increased risk of GI SMN (95%CI=2.5-36.5) among allogeneic BMT recipients; the association was primarily driven by pancreatic and esophageal cancer. Chemotherapeutic agents: Pre-BMT anthracyclines (HR=4.84, 95%CI=1.2-20.4) and etoposide conditioning (HR=2.87, 95%CI=1.6-5.3) were associated with an increased risk of liver cancer. Cytarabine conditioning was associated with increased risk of colorectal cancer (HR=3.08, 95%CI=1.4-6.9). Chronic GvHD: Risk of esophageal cancer was 9.6-fold higher among allogeneic BMT recipients with chronic GvHD (95%CI=3.3-28.4), when compared with those who received an autologous BMT.
Conclusions: This study provides evidence that allogeneic BMT survivors are at increased risk for developing GI SMNs in the setting of specific pre-BMT and BMT-related therapeutic exposures and chronic GvHD. In particular, exposure to myeloablative doses of TBI at age <30y and conditioning with cytarabine and etoposide were associated with increased risk. In addition, pre-BMT exposure to abdominal radiation and anthracyclines were associated with specific types of GI SMNs. These findings and the very high risk of esophageal cancer among those with chronic GvHD provide a framework for developing personalized screening recommendations for those at highest risk.
McDonald: Varian Medical Systems: Consultancy, Research Funding. Weisdorf: Fate Therapeutics: Research Funding; Incyte: Research Funding. Forman: Mustang Bio: Consultancy, Current holder of individual stocks in a privately-held company; Lixte Biotechnology: Consultancy, Current holder of individual stocks in a privately-held company; Allogene: Consultancy. Arora: Syndax: Research Funding; Pharmacyclics: Research Funding; Kadmom: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal